Section: New Results
Human vision understanding through joint experimental and modeling studies, for normal and distrophic vision
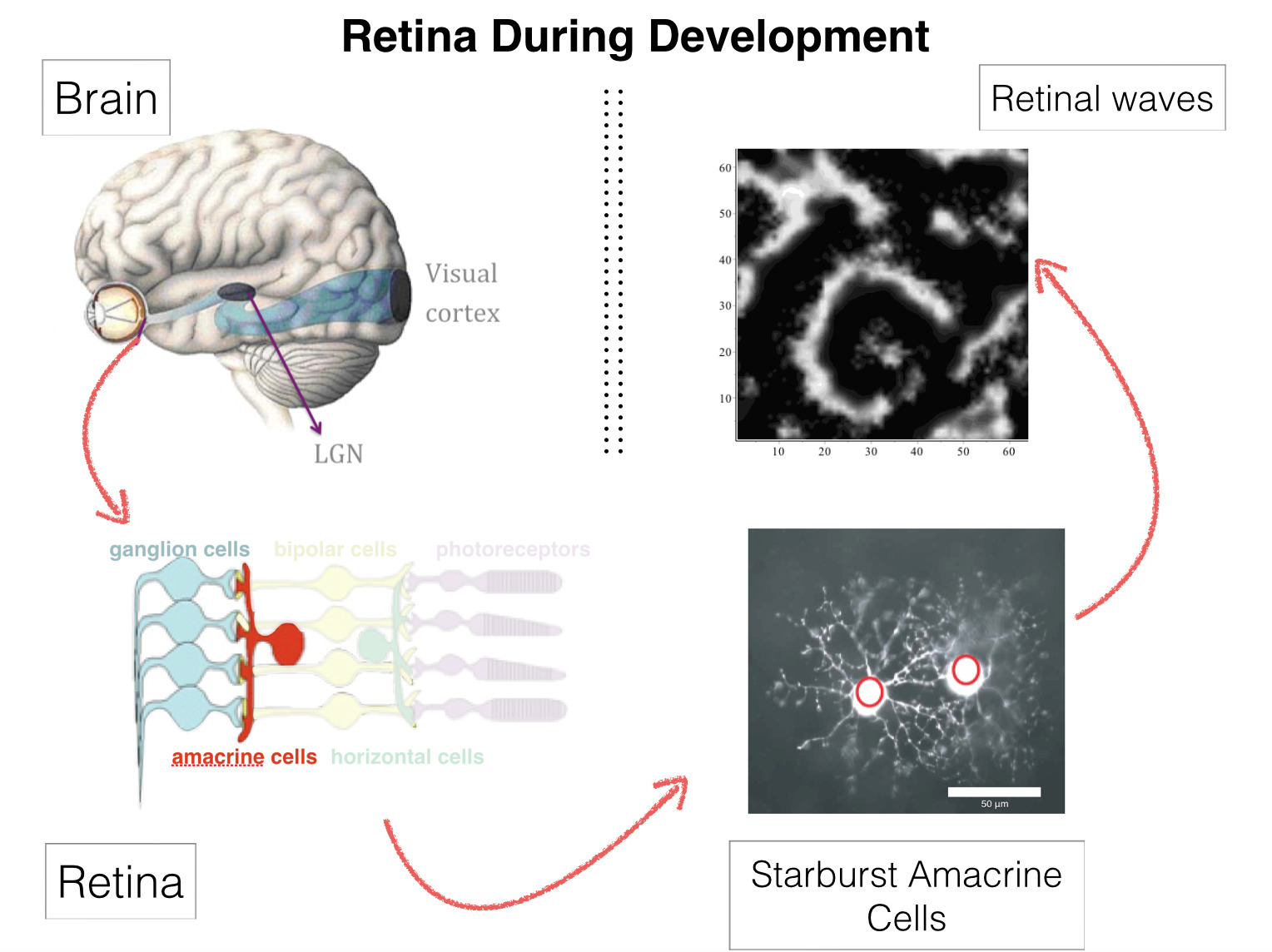
Retinal waves
Participants : Dora Karvouniari, Lionel Gil [Institut Non Linéaire de Nice - Institut de Physique de Nice (INLN, Université Côte d'Azur (France), France)] , Olivier Marre [Institut de la Vision (Paris, France)] , Serge Picaud [Institut de la Vision (Paris, France)] , Bruno Cessac.
Retinal waves are bursts of activity occurring spontaneously in the developing retina of vertebrate species, contributing to the shaping of the visual system organization: retina circuitry shaping, retinotopy, eye segregation [51], [36], [46], [37]. They stop a few weeks after birth. Wave activity begins in the early development, long before the retina is responsive to light. It was recently found that they can be reinitiated pharmacologically in the adult mammalian retina [35]. This could have deep consequences on therapy for several degenerative retinal diseases. The mechanism of their generation, in developing, or adult retinas, remains however incompletely understood [52].
We have proposed a model for stage II retinal waves - induced by bursting Starburst Amacrine Cells (SACs) coupled by acetylcholine - with two objectives: (i) being sufficiently close to biophysics to explain and propose experiments and (ii) affording a mathematical analysis. From a bifurcations analysis we have highlighted several relevant biophysical parameters controlling waves generation, mainly regulating potassium and calcium dynamics. We thus explain how SACs in different species exhibit a large variability in their bursting periods with a common mechanism. We have proposed a testable experimental prediction providing a possible link of the evolution of voltage-dependent potassium channels along development with their role on the excitability properties of SACs. We have reproduced experimental findings (statistical characteristics of waves size, duration and frequency of appearance) and analysed how the evolution of cholinergic conductance due to the maturation of nicotinic receptors dramatically changes the retinal wave characteristics. We have also shown that the nonlinear dynamics generates heterogeneous local spatial structures inside which retinal waves propagate. This induces a wide variability in waves characteristics even though the network is perfectly homogeneous.
This work has been presented in [11], [13], [14], [16], [20], [12], [24].
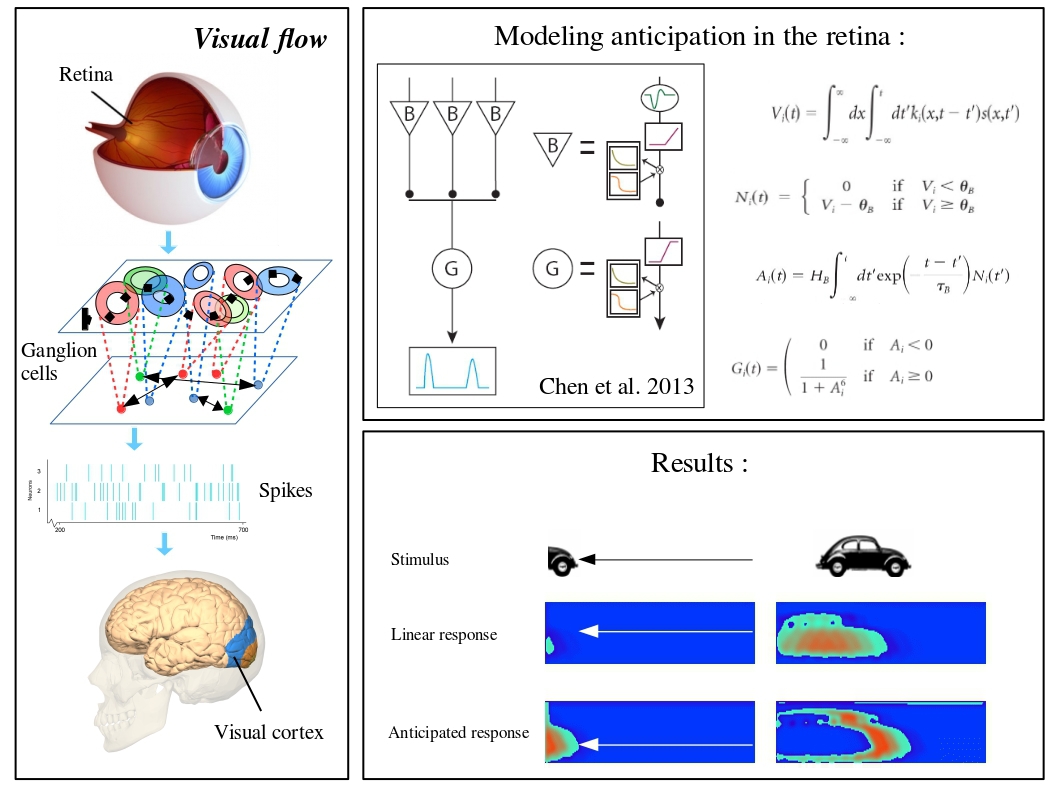
Trajectory anticipation, from retina to V1
Participants : Bruno Cessac, Selma Souihel, Frédéric Chavane, Alain Destexhe, Matteo Di Volo, Olivier Marre.
Global motion processing is a major computational task of biological visual systems. When an object moves across the visual field, the sequence of visited positions is strongly correlated in space and time, forming a trajectory. These correlated images generate a sequence of local activation of the feedforward stream. At the present stage of knowledge, it is still unclear how the early visual system processes motion trajectories. Motion integration, anticipation and prediction would be jointly achieved through the interactions between feed-forward, lateral and feedback propagations within a common spatial reference frame, the retinotopic maps. Addressing this problem is particularly challenging, as it requires to probe these sequences of events at multiple scales (from individual cells to large networks) and multiple stages (retina, primary visual cortex V1).
In the context of the ANR Trajectory, we are working on such an integrated approach. We aim at modelling the population responses at two key stages of visual motion encoding: the retina and V1 based on simultaneous micro- and mesoscopic recordings made by our partners Institut de Neurosciences de la Timone (CNRS and Aix-Marseille Université, France), Institut de la Vision (Paris, France) and Unité de Neurosciences Information et Complexité, Gif sur Yvette, France. We are designing a simulator of retinal output + V1, reproducing both the retinal anticipation and the cortical response measured by optical imaging. We are also analyzing the effects of lateral connectivity in the retina, via amacrine cells, in processing motion. This lateral connectivity is accountable for the correlated activity of RGCs in experimental data. We are measuring these correlations to add further biological plausibility to our model. This study is a step toward understanding mechanisms of motion coding and anticipation with strong impact on our understanding of the visual system.
These results have been presented in [26], [27]
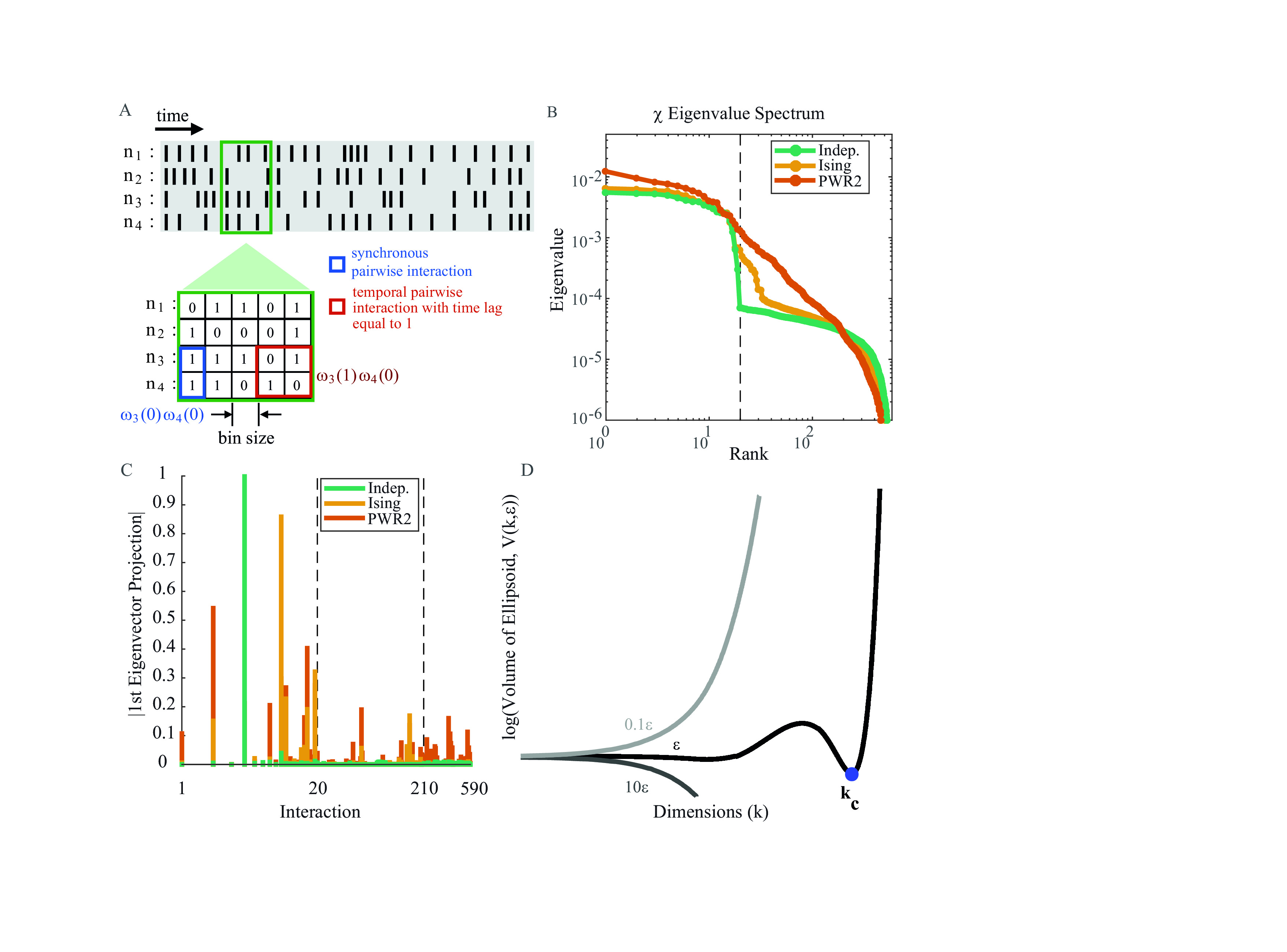
Dimensionality reduction in spatio-temporal MaxEnt models and analysis of retinal ganglion cell spiking activity in experiments
Participants : Rubén Herzog [Centro Interdisciplinario de Neurociencia de Valparaíso (CINV, Valparaíso, Chile)] , Rodrigo Cofré [Centro Interdisciplinario de Neurociencia de Valparaíso (CINV, Valparaíso, Chile)] , Maria-Jose Escobar [Universidad Tecnico Federico Santa María (Electronics Engineering Department, Valparaíso, Chile)] , Adrian Palacios [Centro Interdisciplinario de Neurociencia de Valparaíso (CINV, Valparaíso, Chile)] , Bruno Cessac.
Retinal spike response to stimuli is constrained, on one hand by short range correlations (receptive field overlap) and on the other hand by lateral connectivity (cells connectivity). This last effect is difficult to handle from statistics because it requires to consider spatio-temporal correlations with a time delay long enough to take into account the time of propagation along synapses. Although MaxEnt models are useful to fit optimal model (maximizing entropy) under the constraints of reproducing observed correlations, they do address spatio-temporal correlations in their classical form (Ising or higher order interactions but without time delay). Binning in such models somewhat integrates propagation effects, but in an implicit form, and increasing binning severely bias data. To resolve this issue we have considered spatio-temporal MaxEnt model formerly developed e.g. by Vasquez et al. [49]. The price to pay, however is a huge set of parameters that must be fitted to experimental data to explain the observed spiking patterns statistics. There is no a priori knowledge of which parameters are relevant and which ones are contributing to overfitting. We propose here a method of dimension reduction, i.e. a projection on a relevant subset of parameters, relying on the so-called Susceptibility matrix closely related to the Fisher information. In contrast to standard methods in information geometry though, this matrix handles space and time correlations. We have applied this method for retina data obtained in a diurnal rodent (Octodon degus, having 30 of cones photoreceptors) and a 252-MEA system. Three types of stimuli were used: spatio-temporal uniform light, white noise and a natural movie. We show the role played by time-delayed pairwise interactions in the neural response to stimuli both for close and distant cells. Our conclusion is that, to explain the population spiking statistics we need both short-distance interactions as well as long-distance interactions, meaning that the relevant functional correlations are mediated not only by common input (i.e. receptive field overlap, electrical coupling; spillover) but also by long range connections.
This work has been submitted to Plos Comp Bio [22].
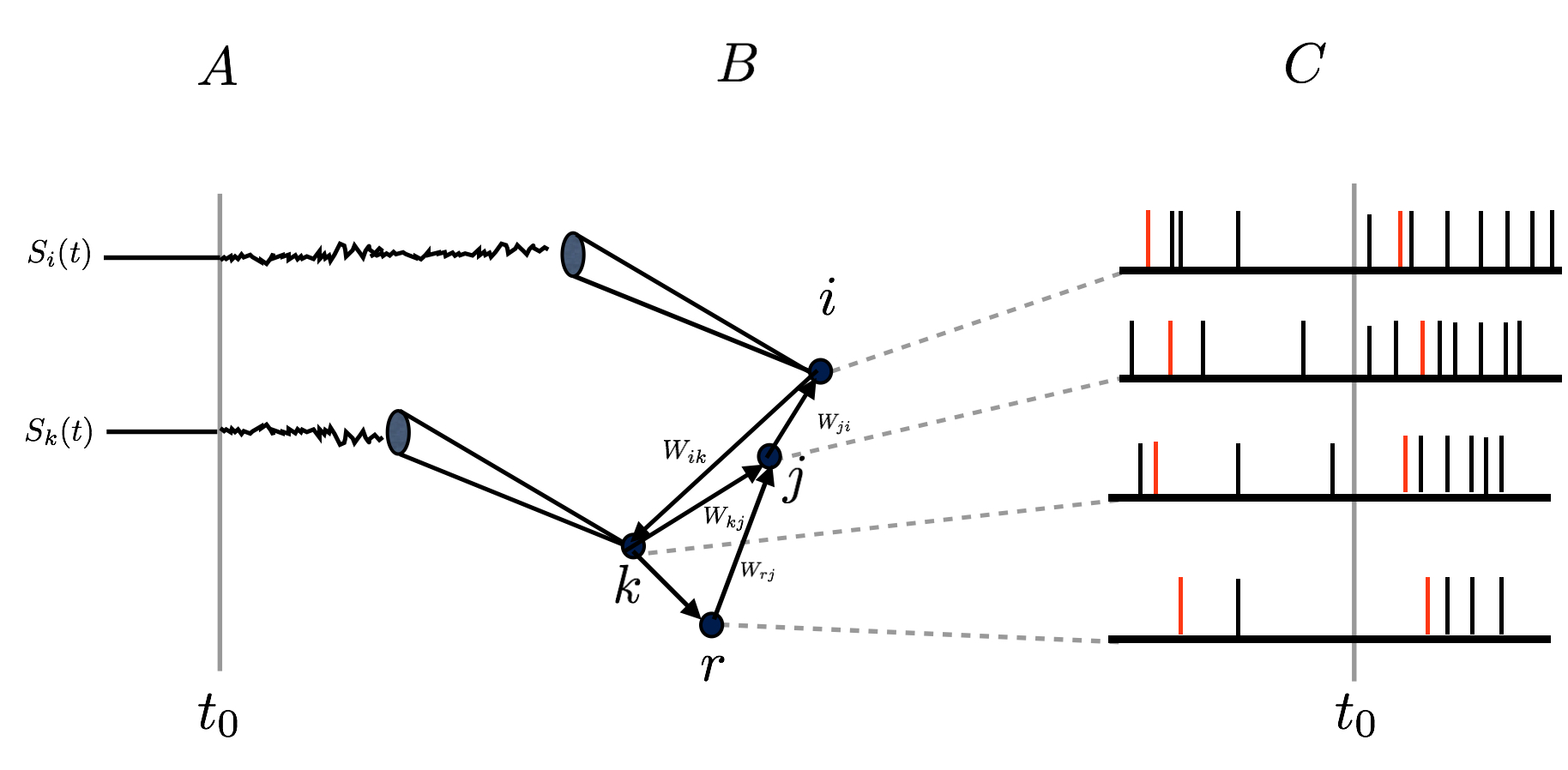
Linear response for spiking neuronal networks with unbounded memory
Participants : Bruno Cessac, Rodrigo Cofré [Centro Interdisciplinario de Neurociencia de Valparaíso (CINV, Valparaíso, Chile)] .
The activity of a neuronal network, characterized by action potentials (spikes), is constrained by the intrinsic properties of neurons and their interactions. When a neuronal network is submitted to external stimuli, the statistics of spikes changes, and it is difficult to disentangle the influence of the stimuli from the intrinsic dynamics. We have established a general linear response relation for spiking neuronal networks, based on chains with unbounded memory. This relation allows quantifying the influence of a weak amplitude external stimuli on spatio-temporal spike correlations, in a general context where the memory in spike dynamics can go arbitrarily far in the past. With this approach, we show how linear response is explicitly related to neuron dynamics with an example, the gIF model, introduced by M. Rudolph and A. Destexhe [91]. This illustrates the effect of the stimuli, intrinsic neuronal dynamics, and network connectivity on spike statistics.
|
This work has been submitted to Journal of Mathematical Neurosciences [19].